Introduction To Chemical Reactions and Equation
CHEMICAL REACTION
A chemical reaction is a process in which one or more substances, also called reactants, are converted to one or more different substances, known as products.
For example: Rusting of iron, the setting of milk into curd, digestion of food, respiration, etc.
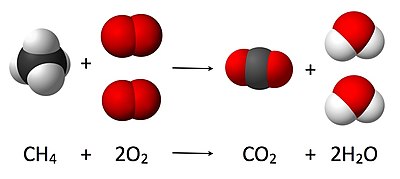
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products.
SOME EXAMPLES OF CHEMICAL REACTION
- Combustion.
- Oxidation (rusting)
- Decomposition or fermentation.
- Cooking an egg.
- Photosynthesis.
- Reacting acids and bases together.
- Chemical batteries.
- Digestion.
One of the Best contents on Internet
ReplyDelete